- Announcing the 2030 Census Disclosure Avoidance Research Program
- CMS: Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and Policy Changes and Fiscal Year 2026 Rates; Requirements for Quality Programs; and Other Policy Changes; Correction
- CMS: Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and Policy Changes and Fiscal Year 2026 Rates; Requirements for Quality Programs; and Other Policy Changes; Correction
- CMS: Medicare and Medicaid Programs; Contract Year 2026 Policy and Technical Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program, Medicare Cost Plan Program, and Programs of All-Inclusive Care for the Elderly; Correction
- CMS: Medicare and Medicaid Programs; Contract Year 2026 Policy and Technical Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program, Medicare Cost Plan Program, and Programs of All-Inclusive Care for the Elderly; Correction
- CMS: Medicare Program; Prospective Payment System and Consolidated Billing for Skilled Nursing Facilities; Updates to the Quality Reporting Program for Federal Fiscal Year 2026
- CMS: Medicare Program; FY 2026 Hospice Wage Index and Payment Rate Update and Hospice Quality Reporting Program Requirements
- Public Inspection: CMS: Medicare Program: Fiscal Year 2026 Hospice Wage Index and Payment Rate Update and Hospice Quality Reporting Program Requirements
- Public Inspection: CMS: Medicare Program: Prospective Payment System and Consolidated Billing for Skilled Nursing Facilities; Updates to the Quality Reporting Program for Federal Fiscal Year 2026
- CMS: Request for Information; Health Technology Ecosystem
- CMS: Medicare and Medicaid Programs; CY 2025 Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Payment and Coverage Policies; Medicare Shared Savings Program Requirements; Medicare Prescription Drug Inflation Rebate Program; and Medicare Overpayments; and Appeal Rights for Certain Changes in Patient Status; Corrections and Correcting Amendment
- CMS: Medicare and Medicaid Programs; CY 2025 Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Payment and Coverage Policies; Medicare Shared Savings Program Requirements; Medicare Prescription Drug Inflation Rebate Program; and Medicare Overpayments; and Appeal Rights for Certain Changes in Patient Status; Corrections and Correcting Amendment
- VA: Staff Sergeant Fox Suicide Prevention Grant Program Funding Opportunity
- State: 60-Day Notice of Proposed Information Collection: J-1 Visa Waiver Recommendation Application
- HHS: Request for Information (RFI): Ensuring Lawful Regulation and Unleashing Innovation To Make American Healthy Again
Medicare FFS Claims: 2% Payment Adjustment Suspended (Sequestration)

Section 3709 of the Coronavirus Aid, Relief, and Economic Security (CARES) Act temporarily suspends the 2% payment adjustment currently applied to all Medicare Fee-For-Service (FFS) claims due to sequestration. The suspension is effective for claims with dates of service from May 1 through December 31, 2020.
CMS Announces that Using CS Modifier When Cost-Sharing is Waived

This clarifies a prior message that appeared in CMS’ April 7, 2020 Special Edition.
CMS now waives cost-sharing (coinsurance and deductible amounts) under Medicare Part B for Medicare patients for certain COVID-19 testing-related services. Previously, CMS made available the CS modifier for the gulf oil spill in 2010; however, CMS recently repurposed the CS modifier for COVID-19 purposes. Now, for services furnished on March 18, 2020, and through the end of the Public Health Emergency, outpatient providers, physicians, and other providers and suppliers that bill Medicare for Part B services under specific payment systems outlined in the April 7 message should use the CS modifier on applicable claim lines to identify the service as subject to the cost-sharing wavier for COVID-19 testing-related services and to get 100% of the Medicare-approved amount. Additionally, they should NOT charge Medicare patients any co-insurance and/or deductible amounts for those services.
The Giant Company Announces $250,000 Emergency Grant Program to Support Small Businesses During Covid-19 Pandemic
The GIANT Company announced a $250,000 emergency grant program, in partnership with Team Pennsylvania, to support small businesses in Pennsylvania’s food supply chain impacted by the ongoing COVID-19 pandemic.
Applications are now being accepted online through April 24 from any small business involved in growing, making or processing food within the Commonwealth. The GIANT Company and Team Pennsylvania worked with the Pennsylvania Department of Economic and Community Development, the Pennsylvania Department of Agriculture, the Pennsylvania Chamber, and Pennsylvania Food Merchants Association to develop the program.
For additional information on criteria and to apply for an emergency grant from, visit The GIANT Company online. Recipients will be notified in early May.
CMS Updates CAH Swing Bed Guidelines

On March 13, 2020, President Trump declared the 2019 Novel Coronavirus Disease (COVID-19) situation a national emergency, which allows CMS to waive certain federal requirements in the Medicare, Medicaid, and CHIP programs to ensure continued access to quality of care for all Medicare beneficiaries pursuant to section 1135(b) of the Social Security Act (Act). To streamline the section 1135 waiver request and approval process, CMS is continuously issuing a number of blanket waivers for many Medicare provisions, which primarily affect requirements for individual facilities, such as hospitals, long term care facilities, home health agencies, and so on. These waivers are in effect, with a retroactive effective date of March 1, 2020, through the end of the emergency declaration and DO NOT require a request to be sent to the 1135waiver@cms.hhs.gov mailbox or that notification be made to any of CMS’s regional offices.
The Act permits certain small, rural hospitals to enter into a swing bed agreement, under which the hospital can use its beds, as needed, to provide either acute or skilled nursing facility (SNF) care. As defined in the regulations, a swing bed hospital is a hospital or CAH participating in Medicare that has CMS approval to provide post-hospital SNF care and meets certain requirements. To qualify for SNF-level services, a beneficiary must have been hospitalized in a participating or qualified hospital or participating CAH for medically necessary inpatient hospital or inpatient CAH care for at least 3 consecutive calendar days, not counting the date of discharge.
CMS is waiving this 3-day prior hospitalization for coverage of a SNF stay, noted above, in the newly issued rules and waivers. This waiver provides temporary emergency coverage of SNF services without a qualifying hospital stay, for those people who experience dislocations or are otherwise affected by COVID-19. This waiver applies to swing bed hospitals, which are hospitals or CAHs participating in Medicare that have CMS approval to provide post-hospital SNF care and meets certain requirements. See Swing Bed Factsheet located at https://www.cms.gov/Outreachand-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/SwingBedFactsheet.pdf to learn more about hospitals who may provide swing bed services. Currently, there is no waiver modifying the definition of a swing bed hospital and rules that were not amended as a result of 1135 waivers are still in place.
The recently released summary of the “COVID-19 Emergency Declaration Blanket Waivers for Health Care Providers” which is located on the website page at https://www.cms.gov/about-cms/emergency-preparednessresponse-operations/current-emergencies/coronavirus-waivers, is a listing of helpful website resources.
CMS is continuously issuing additional waivers and flexibilities, and it is vital to stay updated.
Please visit https://www.cms.gov/About-CMS/Agency-Information/Emergency/EPRO/Current-Emergencies/Current-Emergencies-page for more of CMS’ actions concerning COVID-19.
Additionally, please browse our MLN Connects at https://www.cms.gov/Outreach-and-Education/Outreach/FFSProvPartProg/Provider-Partnership-Email-Archive to keep abreast of changes and updates impacting the provider community.
Trump Administration Announces Expanded Coverage for Essential Diagnostic Services Amid COVID-19 Public Health Emergency

Agencies Implement Legislation Guaranteeing Coverage of COVID-19 Diagnostic Testing, Including Antibody Testing, and Certain Related Services without Cost Sharing for Enrollees in Private Health Coverage
The Centers for Medicare & Medicaid Services (CMS), together with the Departments of Labor and the Treasury, issued guidance today to ensure Americans with private health insurance have coverage of 2019 Novel Coronavirus (COVID-19) diagnostic testing and certain other related services, including antibody testing, at no cost. As part of the effort to slow the spread of the virus, this guidance is another action the Trump Administration is taking to remove financial barriers for Americans to receive necessary COVID-19 tests and health services, as well as encourage the use of antibody testing that may help to enable health care workers and other Americans to get back to work more quickly.
“It is critical that Americans have peace of mind knowing that cost won’t be a barrier to testing during this national public health emergency,” said Administrator Seema Verma. “Today’s action under the leadership of President Trump allows millions of Americans to access the vital health services they need to fight COVID-19, including antibody testing once it becomes widely available.”
In March, representatives of major health insurance companies met with President Trump, where they voluntarily committed to covering COVID-19 testing without cost sharing such as copays and coinsurance. Building on this commitment, today’s guidance implements the recently enacted Families First Coronavirus Response Act (FFCRA) and Coronavirus Aid, Relief, and Economic Security (CARES) Act, which require that private health issuers and employer group health plans cover COVID-19 testing and certain related items and services furnished during the COVID-19 pandemic, with no out-of-pocket expenses.
Specifically, today’s announcement implements the requirement for group health plans and group and individual health insurance to cover both diagnostic testing and certain related items and services provided during a medical visit with no cost sharing. This includes urgent care visits, emergency room visits, and in-person or telehealth visits to the doctor’s office that result in an order for or administration of a COVID-19 test. Covered COVID-19 tests include all FDA-authorized COVID-19 diagnostic tests, COVID-19 diagnostic tests that developers request authorization for on an emergency basis, and COVID-19 diagnostic tests developed in and authorized by states. It also ensures that COVID-19 antibody testing will also be covered. Once broadly available, a COVID-19 antibody test could become a key element in fighting the pandemic by providing a more accurate measure of how many people have been infected and potentially enabling Americans to get back to work more quickly.
To see the guidance, visit: https://www.cms.gov/files/document/FFCRA-Part-42-FAQs.pdf
This action, and earlier CMS actions in response to COVID-19, are part of the ongoing White House Coronavirus Task Force efforts. To keep up with the important work the Task Force is doing in response to COVID-19, visit www.coronavirus.gov. For a complete and updated list of CMS actions, and other information specific to CMS, please visit the Current Emergencies Website.
COVID-19 Disease Continues Steady Spread in Rural Areas from April 5-9
Daily Yonder, By Bill Bishop
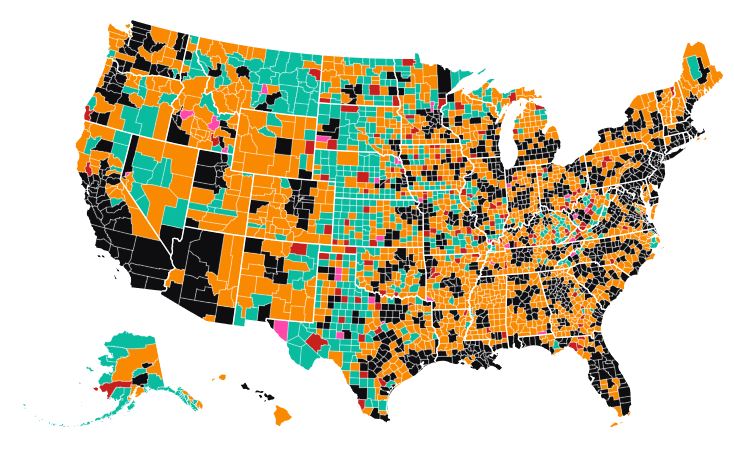
Nearly three out of every four rural counties have officially reported having a case of COVID-19 by the end of Thursday, according to data compiled from state health care agencies by USA Facts.
The map shows the spread of the novel coronavirus through rural America. The 130 rural counties in red reported their first case of COVID-19 between Sunday and Thursday, April 5-9, 2020. Only 31 counties (out of 1,164) metro counties have yet to report their first case of the virus as of the end of Thursday (April 9).
As of April 9, there have been 563 deaths from the virus in rural America. For the past week, the number of deaths from COVID-19 in rural counties has increased 12 to 17 % each day. Deaths in rural counties are increasing at about the same rate as the nation as a whole.
Deaths have been increasing the fastest in the suburbs of the nation’s major metropolitan areas, those with over a million people. Rural America still has a per capita rate of infection that is just a quarter of the national rate.
Click on the map and you can find data for your county. We have included the number of confirmed cases of COVID-19 and the number of deaths as of April 9.
Within the national picture of spreading cases and deaths are local stories that explain why some places have suffered more from the virus than others. For instance, we noticed that Mitchell County, Georgia, reported 12 deaths between Sunday and Thursday. News reports tells us that there was an outbreak of the virus at a nursing home, infecting 26 residents and killing nine in the last week.
There was also an outbreak at a Tyson Foods plant in Camilla, the county seat. Two Tyson workers have died after testing positive for COVID-19, according to the Retail, Wholesale and Department Store Union, which represents workers at the plant. Many workers at the Camilla plant commute from Albany, Georgia, which has had a large outbreak of the virus stemming from a funeral.
Kent County, Delaware, reported the largest number of new cases of COVID-19 in the last week of any rural county, with 128. It was followed by Litchfield County, Connecticut, with 118 and Sumter County, Georgia, with 111.
The COVID-19 pandemic is a world-wide event, but every community is experiencing it differently. Please tell us what’s happening in your community in the comments below or on our Facebook page. We can learn from each other.
USDA Unveils Tool to Help Rural Communities Address the COVID-19 Pandemic
USDA’s COVID-19 Federal Rural Resource Guide Lists Federal Programs That Can Help Rural Communities, Organizations and Residents Impacted by COVID-19
WASHINGTON, April 13, 2020 – U.S. Secretary of Agriculture Sonny Perdue today unveiled a one-stop-shop of federal programs that can be used by rural communities, organizations and individuals impacted by the COVID-19 pandemic. The COVID-19 Federal Rural Resource Guide (PDF, 349 KB) is a first-of-its-kind resource for rural leaders looking for federal funding and partnership opportunities to help address this pandemic.
“Under the leadership of President Trump, USDA is committed to being a strong partner to rural communities preparing for and impacted by COVID-19,” Perdue said. “This resource guide will help our rural leaders, whether they are in agriculture, education, health care or any other leadership capacity, understand what federal assistance is available for their communities during this unprecedented time.”
USDA has taken many immediate actions to assist farmers, ranchers, producers, rural communities, and rural-based businesses and organizations impacted by the COVID-19 pandemic. For more information on these actions, visit www.usda.gov/coronavirus.
Pennsylvania Has the 3rd Least Affected Small Businesses Due to Coronavirus – WalletHub Study
With 35 percent of small business owners saying their business cannot survive more than three months in current conditions, WalletHub today released its report on the States with the Most Affected Small Businesses due to Coronavirus, along with accompanying videos.
To identify the states in which businesses are hit hardest by COVID-19, WalletHub compared the 50 states and the District of Columbia across 12 key metrics. Our data set ranges from the share of small businesses operating in highly affected industries to small-business credit conditions and the state’s small-business friendliness. Below, you can see highlights from WalletHub’s report and a Q&A with WalletHub analysts.
COVID-19 Impact on Small Business in Pennsylvania (1=Most Affected, 25=Avg.):
- 43rd – Share of Small Businesses Operating in High-Risk Industries
- 51st – Share of Small-Business Employees Operating in High-Risk Industries Among Total Small-Business Employees
- 35th – Share of Consumer Expenditures Related to High-Risk Industries
- 20th – Share of Businesses with E-commerce Sales Activity
- 36th – Business Vitality
- 37th – Average Annual Federal Small-Business Funding per GDP
- 30th – Small-Business Credit Conditions
- 33rd – Total Amount of Small-Business Loans per Small-Business Employee
To view the full report and your state’s rank, please visit:
https://wallethub.com/edu/states-with-the-most-affected-small-businesses-due-to-coronavirus/72977/
USDA Announces Loan Maturity for Marketing Assistance Loans Now Extended to 12 Months
Agricultural producers now have more time to repay Marketing Assistance Loans (MAL) as part of the U.S. Department of Agriculture’s implementation of the Coronavirus Aid, Relief, and Economic Security (CARES) Act of 2020. The loans now mature at 12 months rather than nine, and this flexibility is available for most commodities.
Eligible commodities include barley, chickpeas (small and large), corn, cotton (upland and extra-long staple), dry peas, grain sorghum, honey, lentils, mohair, oats, peanuts, rice (long and medium grain), soybeans, unshorn pelts, wheat, wool (graded and nongraded); and other oilseeds, including canola, crambe, flaxseed, mustard seed, rapeseed, safflower, sunflower seed, and sesame seed. Seed cotton and sugar are not eligible
Pennsylvania Issues 30 Licensing Waivers Allowing Professionals to Respond to COVID-19 Emergency
Harrisburg, PA – The Pennsylvania Department of State announced that it has issued 30 licensing waivers since March 17 to allow licensed professionals, facilities and trainees to respond to the COVID-19 disaster declaration.
“During this unprecedented emergency, the Department of State is committed to reducing as many burdens as possible for licensees to practice and serve Pennsylvanians,” Secretary of State Kathy Boockvar said. “We’ve included a wide spectrum of professionals in these temporary waivers, recognizing that each professional we can empower to help is another critical part of the solution to ending this crisis.”
Among the changes in place during the COVID-19 emergency:
- Licensed health care practitioners may provide services via telemedicine
- Temporary licenses for out-of-state health care practitioners will be expedited
- Extended all upcoming license renewal deadlines including healthcare and non-healthcare professionals
- Recently retired health care practitioners may temporarily reactivate their licenses more easily and without reactivation fees
- Suspended certain in-person continuing-education requirements to allow increased use of online or distance learning
- Authorized the use of electronic notarization and loosened restrictions on in person requirements for notaries handling estate documents such as wills, living wills, and powers of attorney, as well as other types
- Extended filing deadlines for charitable nonprofit organizations
“The Department of State is working with the governor’s office, the Department of Health and the Department of Human Services to identify additional requirements that can be suspended to give licensed professionals and others the flexibility they need during the COVID-19 pandemic,” Secretary Boockvar said.
The Department of State website will be updated regularly as additional requirement waiver information becomes available. Licensees with questions should contact their state licensing board via the email addresses on the Department of State website.
