- Public Inspection: CMS: Medicare Program: Implementation of Prior Authorization for Select Services for the Wasteful and Inappropriate Services Reduction Model
- CMS: Secretarial Comments on the CBE's (Battelle Memorial Institute) 2024 Activities: Report to Congress and the Secretary of the Department of Health and Human Services
- HHS: Patient Protection and Affordable Care Act: Marketplace Integrity and Affordability
- HRSA Announces Action to Lower Out-of-Pocket Costs for Life-Saving Medications at Health Centers Nationwide
- Public Inspection: HHS: Patient Protection and Affordable Care Act: Marketplace Integrity and Affordability
- Increased Risk of Cyber Threats Against Healthcare and Public Health Sector
- Eight Hospitals Selected for First Cohort of Rural Hospital Stabilization Program
- Announcing the 2030 Census Disclosure Avoidance Research Program
- CMS: Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and Policy Changes and Fiscal Year 2026 Rates; Requirements for Quality Programs; and Other Policy Changes; Correction
- CMS: Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and Policy Changes and Fiscal Year 2026 Rates; Requirements for Quality Programs; and Other Policy Changes; Correction
- CMS: Medicare and Medicaid Programs; Contract Year 2026 Policy and Technical Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program, Medicare Cost Plan Program, and Programs of All-Inclusive Care for the Elderly; Correction
- CMS: Medicare and Medicaid Programs; Contract Year 2026 Policy and Technical Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program, Medicare Cost Plan Program, and Programs of All-Inclusive Care for the Elderly; Correction
- CMS: Medicare Program; Prospective Payment System and Consolidated Billing for Skilled Nursing Facilities; Updates to the Quality Reporting Program for Federal Fiscal Year 2026
- CMS: Medicare Program; FY 2026 Hospice Wage Index and Payment Rate Update and Hospice Quality Reporting Program Requirements
- Public Inspection: CMS: Medicare Program: Fiscal Year 2026 Hospice Wage Index and Payment Rate Update and Hospice Quality Reporting Program Requirements
Stakeholder Announcement: USDA Implements Immediate Measures to Help Rural Residents, Businesses and Communities Affected by COVID-19: Updated April 8, 2020
WASHINGTON, April 8, 2020 – USDA Rural Development has taken a number of immediate actions to help rural residents, businesses and communities affected by the COVID-19 outbreak. Rural Development will keep our customers, partners, and stakeholders continuously updated as more actions are taken to better serve rural America.
Read the full announcement to learn more about the opportunities USDA Rural Development is implementing to provide immediate relief to our customers, partners, and stakeholders.
RWJF: Highlighting Incarceration as a Key Measure of Health in America
The COVID-19 pandemic has underscored now more than ever how incarceration and health are inextricably linked. The Robert Wood Johnson Foundation (RWJF) has included incarceration among 35 illustrative measures being used to track progress toward building a Culture of Health in America. To further explore incarceration as a key measure of health in the United States, on April 2, the Culture of Health blog published a timely post by RWJF’s Carolyn Miller and Doug Yeung of RAND. The post looks at the important effects of incarceration on health and health equity for prisoners, families and communities.
The post also includes a reference and link to a recent issue of the American Journal of Public Health, supported by RWJF, that sheds light on new research that broadens our understanding of how incarceration negatively influences possibilities of hope, happiness, sense of security, and other critical components of well-being.
Pennsylvania Launches Statewide COVID-19 Support & Referral Helpline
Support & Referral Helpline
The Pennsylvania Department of Human Services (DHS) has launched the statewide Support & Referral Helpline staffed by skilled and compassionate caseworkers who will be available 24/7 to counsel Pennsylvanians struggling with anxiety and other challenging emotions due to the COVID-19 emergency and refer them to community-based resources that can further help to meet individual needs.
“Pennsylvanians will overcome this crisis together by following the guidance of public health professionals who advise social distancing to slow the spread of the COVID-19 virus, but physical isolation does not mean social isolation,” said DHS Secretary Teresa Miller. “We must support people where they are during this time of crisis.”
The toll-free, round-the-clock support line is available at
1-855-284-2494. For TTY, dial 724-631-5600.
To create and staff the support line, DHS has partnered with the Center for Community Resources (CCR), an experienced regional crisis and call center provider based in Butler County, licensed to provide crisis services.
CCR staff are trained to be accessible, culturally competent and skilled at assisting individuals with mental illness, intellectual disabilities, co-occurring disorders and other special needs. Staff use the principles of trauma-informed care to listen, assess needs, triage calls, and provide appropriate referral to community resources to children, teens, adults and special populations.
CCR will collaborate with individuals, families, police, emergency medical teams, hospitals, schools, and human service providers on the local level to provide quality care to their community members.
“We recognize the significant strain this crisis is putting on families across Pennsylvania, and we want you to know that you do not have to struggle alone. If you need help, reach out,” said Secretary Miller. “The compassionate caseworkers staffing the Support & Referral Helpline will be there to answer your call and be a line of support during this difficult time.”
Many other resources also remain available to Pennsylvanians in need of support, including:
- National Suicide Prevention Lifeline: 1-800-273-TALK (8255)
- Nacional de Prevención del Suicidio: 1-888-628-9454
- Crisis Text Line: Text “PA” to 741-741
- Veteran Crisis Line: 1-800-273-TALK (8255)
- Disaster Distress Helpline: 1-800-985-5990
- Get Help Now Hotline (for substance use disorders): 1-800-662-4357
- United Way of Pennsylvania: Text your zip code to 898-211 for resources and information in your community.
For the latest information on COVID-19 in Pennsylvania, visit the Pennsylvania Department of Health website.
USDA Expands Payment Deferrals for Agency Guaranteed Loan Programs
USDA Rural Development Deputy Under Secretary Bette Brand today announced that USDA is expanding servicing options for guaranteed lenders due to the COVID-19 pandemic. Apparently, USDA is expanding upon the deferral flexibility it announced March 31, 2020. More details on the Community Facility Loan Guarantee Program.
What Ag Producers Need to Know About COVID19
From the AgriSafe Network
This webinar took place on March 23, 2020 and highlights evidence-based information about COVID19 to help agricultural producers identify strategies for responding on their farm. The intended audience is ag producers, ranchers, farmers, farmworkers, veterinarians, Extension personnel, rural health care providers, and others who work in agriculture. The webinar is available OnDemand!
At the end of the webinar, participants will be able to:
- Be aware of common signs and symptoms of COVID-19
- Understand the transmission risk to yourself, employees, and potentially your animals
- Describe infection control principles and appropriate strategies for limiting disease transmission
- Locate resources and training for ag producers related to infection prevention.
|
Pennsylvania Guidance on Hospitals’ Responses to COVID-19: Updated April 2, 2020
The Pennsylvania Department of Health (Department) has received questions and requests for guidance from hospitals, health systems, and their representatives on their responses to Coronavirus Disease-2019 (COVID-19) and whether measures being implemented or contemplated are compliant with the statutory and regulatory requirements under the jurisdiction of the Department. The Department is providing the guidance as an update to the guidance issued on March 21, 2020.
UPDATED Guidance on Hospital Responses to COVID-19
Pockets of Rural America Are Less Vulnerable to Economic Fallout — For Now
Daily Yonder
Every part of the country will feel the economic fallout from the coronavirus crisis. But the small and isolated rural areas that lagged during the economic boom may fare better, relatively speaking, in the aftermath of the pandemic.
Those places tend to be less tied to global and financial markets. With little population density, they are less conducive to virus transmission. So far, states such as Wyoming, the Dakotas, Nebraska and Iowa have reported far fewer COVID-19 cases than New York and other states with large cities.
“If you are a somewhat more isolated economy that does not attract as much visitation from either outside the U.S. or even domestically, you are less vulnerable,” said Adam Kamins, an economist and director at Moody’s Analytics, in a webinar last month.
The states least affected by the huge spike in unemployment claims are largely rural. They include West Virginia, Arkansas and Georgia. In part, that’s because those states have taken less dramatic steps to slow the spread of the virus. Among them, only West Virginia issued a stay-at-home order before the end of March.
Nevertheless, “the industries that have been hard hit are just not as prevalent in rural areas,” said Ernie Goss, an economics professor at Creighton University in Omaha, Nebraska. He cited the relative lack of retail and hospitality businesses in Corn Belt states.
Economists rank regions as economically vulnerable to coronavirus fallout based on demographic and economic factors, including their number of COVID-19 cases, connection to international travelers, reliance on tourism, population density and reliance on global trade, according to a Moody’s Analytics analysis.
171 Rural Counties Report First Case of COVID-19 in Past Four Days
Daily Yonder
Coronavirus Infection Rate, April 5
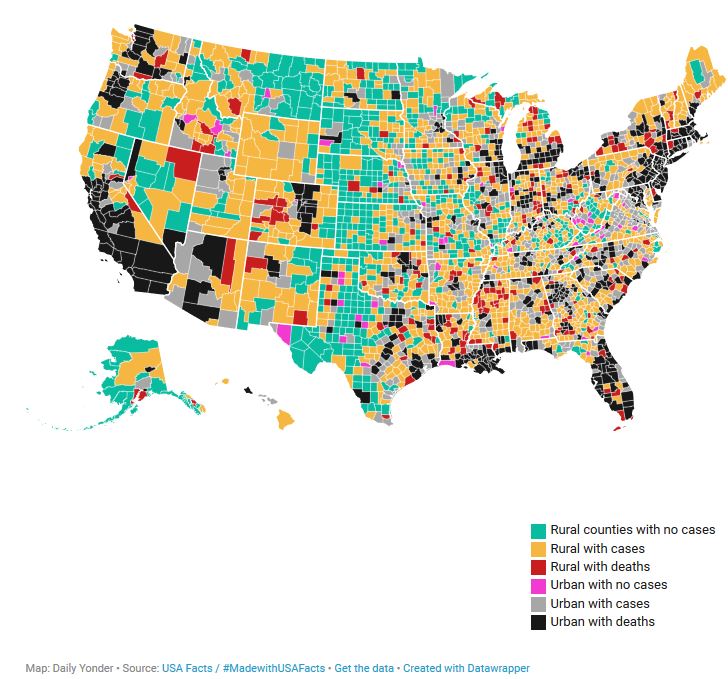
The novel coronavirus continued its march across rural America over the weekend. By Sunday night, April 5, two-thirds of rural counties had at least one case. Just over 200 rural counties have reported a death attributable to COVID-19.
The map above shows the spread of the virus and deaths as of Sunday night, April 5. Click on individual counties for more information, or explore a larger version of the map here.
- Green: Rural counties with no cases (665 counties)
- Orange: Rural counties with cases of COVID-19 (1,109 counties)
- Red: Rural counties with deaths (203 counties)
- Pink: Urban with no cases (61 counties)
- Gray: Urban with cases (575 counties)
- Black: Urban with deaths (528 counties)
These figures likely under-report the presence of the disease, according to a study by researchers at the University of Texas. They estimate that even in counties that report no COVID-19 cases, there is a 9 percent chance that the virus is present in that community.
If a county has one case, the Texas researchers predict that there is a 51 percent chance that the virus is spreading through the community.
From April 1 to 5, an additional 172 rural counties reported a case of coronavirus infection. Only 665—or about a third—of rural counties have yet to report a case of COVID-19. Only 61 urban counties—5 percent of all metro counties—say they have yet to find a COVID-19 case.
The number of reported COVID-19 cases continues to increase slightly faster in rural counties than in urban areas. Over the weekend (Friday through Sunday, April 3-5), cases in rural counties increased by 26 percent. Nationally, COVID-19 cases increased by 22 percent in the same time period.
In rural counties, there were 80 deaths reported over the weekend attributed to COVID-19.
‘Being From A Small Town, You Think It’s Not Going To Touch Us’: Rural America Unprepared For Fast-Spreading Virus
Parts of rural America aren’t seeing the booms like in New York, D.C., and other urban areas, but cases in those parts of the country are now speeding up. Yet, more remote areas also tend to be the places that are already struggling in terms of what their health systems can bear.
Click here to see maps from the New York Times on the spread of COVID-19 in rural America.
National Labor Exchange Launches Job Resource to Support Displaced Workers during Coronavirus Pandemic
Nonprofits DirectEmployers Association and the National Association of State Workforce Agencies (NASWA), announced the launch of NeedAJobNow.USNLx.com, a job site dedicated to providing a centralized location for displaced workers to access employment opportunities from U.S. corporations with immediate hiring needs due to the novel coronavirus (COVID-19). Powered by the National Labor Exchange (NLx), the site houses jobs from vetted employers in all industries and provides an opportunity for Americans to return to work and gain meaningful employment.
NeedAJobNow.USNLx.com contains over 400,000 job openings and continues to grow daily. While many employers are downsizing their staff, others are significantly increasing their hiring efforts due to current demands. Through this initiative, DirectEmployers and NASWA will assist in bridging the gap between job supply and demand by offering an easy way for job seekers to gain access to current open positions and for employers to fill positions quickly and efficiently during these difficult times.
