- CMS: Medicare Program; Implementation of Prior Authorization for Select Services for the Wasteful and Inappropriate Services Reduction (WISeR) Model
- Public Inspection: CMS: Medicare Program: Implementation of Prior Authorization for Select Services for the Wasteful and Inappropriate Services Reduction Model
- CMS: Secretarial Comments on the CBE's (Battelle Memorial Institute) 2024 Activities: Report to Congress and the Secretary of the Department of Health and Human Services
- HHS: Patient Protection and Affordable Care Act: Marketplace Integrity and Affordability
- HRSA Announces Action to Lower Out-of-Pocket Costs for Life-Saving Medications at Health Centers Nationwide
- Public Inspection: HHS: Patient Protection and Affordable Care Act: Marketplace Integrity and Affordability
- Increased Risk of Cyber Threats Against Healthcare and Public Health Sector
- Eight Hospitals Selected for First Cohort of Rural Hospital Stabilization Program
- Announcing the 2030 Census Disclosure Avoidance Research Program
- CMS: Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and Policy Changes and Fiscal Year 2026 Rates; Requirements for Quality Programs; and Other Policy Changes; Correction
- CMS: Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the Long-Term Care Hospital Prospective Payment System and Policy Changes and Fiscal Year 2026 Rates; Requirements for Quality Programs; and Other Policy Changes; Correction
- CMS: Medicare and Medicaid Programs; Contract Year 2026 Policy and Technical Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program, Medicare Cost Plan Program, and Programs of All-Inclusive Care for the Elderly; Correction
- CMS: Medicare and Medicaid Programs; Contract Year 2026 Policy and Technical Changes to the Medicare Advantage Program, Medicare Prescription Drug Benefit Program, Medicare Cost Plan Program, and Programs of All-Inclusive Care for the Elderly; Correction
- CMS: Medicare Program; Prospective Payment System and Consolidated Billing for Skilled Nursing Facilities; Updates to the Quality Reporting Program for Federal Fiscal Year 2026
- CMS: Medicare Program; FY 2026 Hospice Wage Index and Payment Rate Update and Hospice Quality Reporting Program Requirements
Pennsylvania Governor’s Administration Encourages Residents to Support Local Restaurants with CarryoutPA.com
The Pennsylvania Department of Community and Economic Development’s (DCED) Tourism Office encourages Pennsylvanians to support local restaurants by visiting the CarryoutPA website, which offers a comprehensive list of restaurants offering takeout, curbside, or delivery services during the state’s stay-at-home order.
CarryoutPA.com was developed by the Pennsylvania Restaurant and Lodging Association (PRLA) to serve as a go-to resource for dine-out options in support of the commonwealth’s restaurant industry, which accounts for 10 percent of jobs statewide. Pennsylvania restaurants that would like to be added to the registry can register here.
Pennsylvania Department of Agriculture Outlines Updated Farm Labor Requirements, Advocates for Skilled Labor to Ensure Food Supply
The Pennsylvania Department of Agriculture provided farmers who provide housing to their workforce – be it domestic, migrant, or guest H-2A workers – with enhanced requirements for seasonal farm labor to maintain a healthy agriculture workforce to ensure necessary farm labor can continue during COVID-19 mitigation in Pennsylvania. The Wolf Administration continues to advocate for a safe, skilled agriculture workforce to perform essential duties to keep Pennsylvania’s food supply chain strong.
Pennsylvania is home to more than 360 permitted Seasonal Farm Labor Camps with nearly 4,300 workers. The workers in these camps are primarily migrant workers – sourced by their company – or H-2A workers – sourced federally. Even before COVID-19 hit Pennsylvania, the Department of Agriculture’s Bureau of Food safety oversaw the Seasonal Farm Labor Camps where these guest workers reside. The Seasonal Farm Labor Act and regulation sets standards for conditions of work, living quarters, occupancy, camp sanitation, food facilities, fire protection, and safety of farm workers. In addition to these required standards, the department has issued additional requirements for employers to follow to mitigate against COVID-19 for their workforce.
The following are some examples of necessary provisions to maintain the health and safety of seasonal farm workers:
- Per CDC recommendations, there should be a minimum of six feet between beds;
- Beds should be positioned so that workers sleep head-to-toe to limit exposure to respiratory droplets;
- Provide workers with cloth face masks to wear while in housing;
- Ensure bathrooms and other sinks are consistently stocked with soap and drying materials for adequate handwashing;
- Provide hand sanitizer when soap and water are not available;
- Ensure high contact surfaces are cleaned and sanitized on a routine basis with EPA-registered disinfectants;
- Ensure essential supplies for cleaning and sanitizing are available in all living quarters and worksites;
- Designate an individual responsible for maintaining routine cleaning.
In addition to these additional requirements to keep workers from getting sick with COVID-19, the Modified Seasonal Farm Labor Camp Requirements include steps to take if an employee is diagnosed with COVID-19.
CMS Issues Recommendations to Re-Open Health Care Systems in Areas with Low Incidence of COVID-19

As the United States continues to face the unprecedented public health emergency from the COVID-19 pandemic, the tide is turning and some areas throughout the country are seeing a decline in cases. As states and localities begin to stabilize, CMS is issuing guidance on providing essential non-COVID-19 care to patients without symptoms of COVID-19 in regions with low and stable incidence of COVID-19. This is part of Phase 1 in the Trump Administration’s Guidelines for Opening Up America Again. The recommendations update earlier guidance provided by CMS on limiting non-essential surgeries and medical procedures.
COVID-19 Resources for Non-English-Speaking People
The COVID-19 Health Literacy Project provides translations of essential information about COVID-19 in more than 35 languages. Harvard faculty has vetted all of the information in this project that was started by a medical student at Harvard. Patients with limited English proficiency are likely to be at higher risk for COVID-19 and its complications. These translational materials, along with a guidance article, Culturally and Linguistically Competent Care from ECRI, can help provide health centers with the information needed to communicate with those patients whose first language is not English and other patients with diverse cultural needs.
Contact Tracing – Guidance on this Path Out of Isolation
Contact tracing is what is now on everyone’s radar in order to help in the next step to begin to open up the country. Contact tracing is a process designed to halt the chain of transmission of an infectious pathogen–like the coronavirus–and slow community spread. When someone tests positive for an infectious disease, they become a “case.” Public health workers then reach out to the case to make sure they have what they need and that they are self-isolating. They will then figure out who they had contact with who may be at risk of infection, too. NPR recently released a guide on the basics of the process and how it might help society restart after the current wave of coronavirus cases.
US’s digital divide ‘is going to kill people’ as Covid-19 exposes inequalities
Amanda Holpuch, The Guardian
Exclusive research shows drop in connectivity is impacting rural and urban areas with populations already underserved by the medical system or racked with poverty
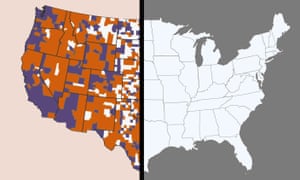
The Covid-19 crisis is exposing how the cracks in the US’s creaking digital infrastructure are potentially putting lives at risk, exclusive research shows.
With most of the country on lockdown and millions relying on the internet for work, healthcare, education and shopping, research by M-Lab, an open source project which monitors global internet performance, showed that internet service slowed across the country after the lockdowns.
“This is going to kill people,” said Sascha Meinrath, co-founder of M-Lab.
In late March, most people in 62% of counties across the US did not have the government’s minimum download speed for broadband internet, according to M-Lab.
Between February and mid March, when the pandemic was only just beginning to hit the US, there was a 10% increase in how many counties saw download speeds fall below the government standard, representing about one in 10 US counties, M-Lab found.
“Now that people’s livelihoods, schools and lives, are literally on the line, we can’t survive,” Meinrath said. “These communities that are underserved are not going to be able to transition to an online workplace or school environment.”
ADA Offers PPE Guidance As States Consider Reopening
The American Dental Association (ADA) has released interim guidance on personal protective equipment (PPE) for states considering reopening dental practices. The interim guidance focuses on the PPE recommended in order to practice during this pandemic and minimize the risk of virus transmission.
Impact of COVID-19 on Dental Practices
The Health Policy Institute (HPI) is conducting a bi-weekly survey of dental providers across the country to gauge the impact of COVID-19 on dental practices. According to the April 6th survey, 29.4% of PA dental practices are closed and not seeing emergency patients during the COVID-19 pandemic. Additionally, 57.2% of PA dental offices are not able to pay their staff at all during this time. A recording of “How is COVID-19 Impacting the Dental Care Sector” is available at the link below and discusses the data.
Click here for the webinar recording.
Click here for more information.
Click here for Pennsylvania-specific information.
Listen: Pandemic Stresses Already Fragile Rural Health Care Systems
Kaiser Health News (KHN) Midwest correspondent Lauren Weber joined WAMU’s “1A” show with guest host Sasha-Ann Simons to talk about the unique challenges rural health care providers face amid the coronavirus pandemic — even before their communities get overrun with a surge of COVID-19 cases.
Weber has reported on how the suspension of elective surgery and other procedures amid the pandemic has threatened the financial survival of the country’s rural hospitals — and how hospital executives don’t feel the first round of federal bailout relief money was enough. She also has written about the front-line fight at a rural Louisiana hospital that forecasts what the pandemic will look like when it hits the rest of rural America.
Listen to the story here.
COVID-19 Outreach and Enrollment News
From the Pennsylvania Association of Community Health Centers (PACHC)
Income and Eligibility for Health Insurance
The Coronavirus Aid, Relief, and Economic Security Act (CARES Act) signed into law on March 27 provided for payments of up to $1200 for every adult and $500 per dependent child. This Stimulus payment does not count as income for Medicaid/CHIP or APTC determinations. It should also not be reported on any application for assistance.
Regular Unemployment is always counted as income. The extension of unemployment of up to 39 weeks and the expansion unemployment to new populations is counted as income. The additional $600/week supplemental unemployment benefit ends July 31, 2020. This benefit does not count toward Medicaid/CHIP but DOES COUNT toward income for Marketplace Coverage and APTC eligibility.
While the Federal government has not issued a special enrollment period or opened the marketplace due to the Pandemic, consumers who have lost coverage may qualify for a special enrollment period. Special Enrollment Period Reference Guide
For more information, visits PACHC’s COVID-19 page.
