- VA Announces Expansion of "Close to Me" Cancer Program as Part of the Cancer Moonshot, Bringing Cancer Diagnosis and Treatment Closer to Thousands of Veterans
- Biden-Harris Administration Releases National Strategy for Suicide Prevention and First-Ever Federal Action Plan
- Biden-Harris Administration Takes Historic Action to Increase Access to Quality Care, and Support to Families and Care Workers
- Biden Administration Sets Higher Staffing Mandates. Most Nursing Homes Don't Meet Them.
- Rural Jails Turn to Community Health Workers To Help the Newly Released Succeed
- Rural Communities Face Primary Care Physician Shortage
- Miles for Milk: How Student-Run Grocery Store Reshaped Rural Community's Food Access
- Native Americans Have Shorter Life Spans, and It's Not Just Due to Lack of Health Care
- Promotoras Play Essential Role in Connecting Farmworkers with Health Care in Rural NorCal
- Across the Country, Amish Populations Are on the Rise
- Using Medicaid to Address Young People's Mental Health Needs in School Settings
- Sunsets, Wildlife and Limited Care: Challenges of Aging in Place in Rural America
- City-Country Mortality Gap Widens amid Persistent Holes in Rural Health Care Access
- Tribal Environmental Impact Network
- Minnesota's Rural Ambulance Providers Look to State Capitol for Their Own Lifeline
Trump Declares COVID-19 Emergency, Asks Hospitals to Activate Emergency Plans
On March 16, 2020, President Donald Trump asked hospitals to activate their emergency preparedness plans and declared the COVID-19 outbreak a national emergency, which will allow HHS to give providers more flexibility. “I’m also asking every hospital in this country to activate its emergency preparedness plan so that they can meet the needs of Americans everywhere,” Trump said.
Ambassador Debbie Birx, the Trump administration’s coronavirus response coordinator, said that hospital emergency preparedness plans could include delaying elective procedures to ensure availability of hospital beds.
The emergency declaration, in conjunction with the administration’s prior designation of COVID-19 as a public health emergency on January 31, frees up to $50 billion in federal disaster relief funding, Trump said, and provides the HHS secretary with more authority to waive some Medicare, Medicaid and Children’s Health Insurance Program (CHIP) requirements.
Trump highlighted that the HHS secretary will have authority to waive several requirements hospitals had voiced concerns about, including the 3-day hospital stay requirement for skilled nursing facility coverage; limits on numbers of beds and length of stay in Critical Access Hospitals; requirements that providers have a license in the state in which they are providing services if they have an equivalent license in another state; HIPAA requirements that could be an obstacle to telemedicine accessibility; and easing restrictions on where certain patients can be treated within a hospital.
“We’ll remove or eliminate every obstacle necessary to deliver our people the care that they need, and that they’re entitled to. No resource will be spared, none whatsoever,” Trump said.
It is unclear precisely how much money would be available for which purposes, but the government has wide discretion in how federal emergency funds are spent, said Georgetown University adjunct law professor and Avalere consultant Nick Diamond.
“The law affords a lot of flexibility for funds to be used in concert with some of the waivers, so there is a possibility for reimbursement for services provided by providers in some of their response efforts,” Diamond said.
The American Hospital Association, American Medical Association and American Nurses Association on Thursday wrote a letter to Vice President Mike Pence, who heads the White House coronavirus response effort, asking for an emergency declaration.
“Physicians, nurses, first responders, and other healthcare professionals across the country are on the front lines in this effort, and streamlining critical processes is vitally important to prevent the further spread of COVID-19,” AMA President Dr. Patrice Harris said in a statement.
Trump also said the administration is in discussions with pharmacies and retailers to set up drive-through testing sites in locations determined by public health officials.
Sen. Rick Scott (R-Fla.), a former Florida governor and health system executive, said he told President Donald Trump on a phone call Thursday that Scott wanted an emergency declaration to provide funding for mobile COVID-19 testing sites to minimize exposure for healthcare workers in primary care and hospital settings who may not have sufficient personal protective equipment.
“I have talked to a lot of hospitals inside and outside the state, and their main concern is how do we make sure they don’t lose their workers?” Scott said.CMS Administrator Seema Verma said Friday that the agency will issue guidance instructing nursing homes to restrict all visitors and non-essential personnel, with exceptions including end-of-life situations.
Senate Democrats had also called upon the administration to declare a national emergency under the Stafford Act.
“Calling for a national emergency under the Stafford Act would free up lots of FEMA’s resources to help states and localities. Why he hasn’t done it is a mystery. We need him to do it, and do it now,” Senate Minority Leader Chuck Schumer (D-N.Y.) said on the Senate floor on Thursday.
Additional guidance on what types of waivers the Trump administration will allow is expected soon. Similar authorities to those outlined by Trump were invoked in the 2009 H1N1 pandemic.
HHS COVID-19 Primary Resources for Faith and Community Organizations
- For updates on the Novel Coronavirus Disease 2019 (COVID-19), refer to the Centers for Disease Control and Prevention’s (CDC’s) dedicated website. Also available in Spanish. CDC.gov/Coronavirus/2019-ncov
- For local information and for recommendations on community actions designed to limit exposure to COVID-19, check with your state and local public health authorities https://go.usa.gov/xdFg4
- For guidance and instruction on specific prevention activities relative to your faith community’s tradition and practices, refer to your national and regional denominations.
Additional Resources
- Implementation of Mitigation Strategies for Communities with Local COVID-19 Transmission.
- Interim Guidance: Get Your Mass Gatherings or Large Community Events Ready for Coronavirus Disease 2019 (COVID-19)
- Interim Guidance: Get Your Community- and Faith-Based Organizations Ready for Coronavirus Disease 2019 (COVID-19)
- Preventing COVID-19 Spread in Communities including guidance for homes, schools, and workplaces
- Track efforts by the federal government, the following websites have been launched:
- English: USA.gov/Coronavirus
- Spanish: https://gobierno.USA.gov/Coronavirus
Guidance to Prepare Homeless Shelters
People experiencing homelessness are an especially vulnerable population. CDC released guidance on March 9, to help homeless shelters plan, prepare, and respond to COVID-19. The guidance can be found here.
Guidance for School Settings UPDATED
CDC’s Coronavirus Disease-2019 (COVID-19) interim guidance for school settings.
CMS: Pricing for CDC and Non-CDC COVID-19 Testing
On March 16, 2020, the Centers for Medicare & Medicaid Services (CMS) is posting a fact sheet to the CMS.gov website to aid Medicare providers with information relating to the pricing of both the CDC and non CDC tests. You can find the fact sheet here: https://www.cms.gov/files/document/mac-covid-19-test-pricing.pdf
Additionally, CMS will be tweeting about this new information. Tweets can be found below. We encourage relevant stakeholders to retweet.
- Medicare will be covering #COVID19 tests, and there is generally no copay for original #Medicare – deductible applies. @CMSGov is also permitting #MedicareAdvantage plans to waive cost sharing for these tests.
- #Medicare’s initial payment for the @CDCgov test will be about $36 & non-CDC tests will be around $51. These prices may vary slightly depending on the local Medicare Administrative Contractor (MAC). View the full price by MAC list here: https://www.cms.gov/files/document/mac-covid-19-test-pricing.pdf
Earlier CMS actions in response to the COVID-19 virus, are part of the ongoing White House Task Force efforts. To keep up with the important work CMS is doing in response to COVID-19, please visit the Current Emergencies Website.
CMS: COVID-10 Guidance Issued for Visitors to Nursing Homes
On March 16, 2020, as part of the broader Trump Administration announcement on Friday, the Centers for Medicare & Medicaid Services (CMS) announced critical new measures designed to keep America’s nursing home residents safe from the 2019 Novel Coronavirus (COVID-19). The measures take the form of a memorandum and is based on the newest recommendations from the Centers for Disease Control and Prevention (CDC). It directs nursing homes to significantly restrict visitors and nonessential personnel, as well as restrict communal activities inside nursing homes. The new measures are CMS’s latest action to protect America’s seniors, who are at highest risk for complications from COVID-19. While visitor restrictions may be difficult for residents and families, it is an important temporary measure for their protection.
You can find the press release here: https://www.cms.gov/newsroom/press-releases/cms-announces-new-measures-protect-nursing-home-residents-covid-19
And the Memo Nursing Home Guidance QSO-20-14 –NH here: https://www.cms.gov/files/document/3-13-2020-nursing-home-guidance-covid-19.pdf
This guidance, and earlier CMS actions in response to the COVID-19 virus, are part of the ongoing White House Task Force efforts. To keep up with the important work the Task Force is doing in response to COVID-19 click here www.coronavirus.gov. For information specific to CMS, please visit the Current Emergencies Website
CMS Sends More Detailed Guidance to Providers about COVID-19
March 10, 2020 — The Centers for Medicare and Medicaid Services (CMS) provided additional guidance to home health agencies and dialysis facilities in response to the 2019 Novel Coronavirus (COVID-19) outbreak. The guidance offers information to healthcare workers on the screening, treatment, and transfer procedures to follow when interacting with patients.
Source: Centers for Medicare and Medicaid Services
COVID-19 Response News Alert: CMS Issues Key Protective Mask Guidance for Healthcare Workers
March 10, 2020 — The Centers for Medicare and Medicaid Services (CMS) issued a memorandum to State Survey Agencies on the types of facemasks health care workers may use when in situations involving Novel Coronavirus 2019 (COVID-19). The guidance is part of an effort to ensure a maximum supply of facemasks and respirators are available to health care providers.
Source: Centers for Medicare and Medicaid Services
Pennsylvania Gov Wolf: Medicaid and CHIP Recipients’ COVID-19 Testing and Treatment Resources are Covered
Harrisburg, PA – March 11, 2020. Governor Tom Wolf announced that the state’s Medicaid program, Medical Assistance (MA) and Children’s Health Insurance Program (CHIP), will cover COVID-19 testing and treatment for recipients and is lifting some prior authorization requirements to ease access to necessary testing and treatment. There are no MA or CHIP copayments for laboratory tests for COVID-19. For those services that do have copayments, MA providers may not deny services if a beneficiary is unable to pay the copayment.
“We are prepared to mitigate COVID-19 throughout the commonwealth, and part of this mitigation includes ensuring that anyone who needs to be tested for COVID-19 can access the test,” said Governor Tom Wolf. “No Pennsylvanian should forego testing for any reason, if deemed medically necessary, including fear of what it might cost.”
The MA and CHIP programs will pay for COVID-19 testing when a health care practitioner determines it is needed, and prior authorization is not required. While there is no specific antiviral treatment for COVID-19, the MA and CHIP programs cover a broad range of services that help relieve symptoms.
The Department of Human Services reminds Pennsylvanians that Medicaid enrollment is year-round and if anyone is currently uninsured, they should go to compass.state.pa.us to see if they qualify for Medicaid.
“We are pleased to make this announcement today and thankful to our partners at the Centers for Medicare for Medicaid Services and our managed care organizations for working with us to ensure that anybody who needs to be tested for COVID-19 will have no barriers to the test,” said DHS Secretary Teresa Miller.
Providers and patients can consult the Medicaid FAQ and CHIP FAQ for more information and answers to common questions related to medical assistance coverage and COVID-19 and information on who to contact if consumers need more information.
The Wolf Administration recently released guidance through the Pennsylvania Insurance Department outlining resources available and coverage for COVID-19 testing through commercial health insurers. Read more on common questions related to commercial insurance coverage and COVID-19 here.
Visit the PA Department of Health’s dedicated Coronavirus webpage for the most up-to-date information regarding COVID-19.
MEDIA CONTACT: Lyndsay Kensinger, 717-783-1116
Erin James, 717-425-7606
Pennsylvania Update on COVID-19 in Community
On March 11, 2020, the Pennsylvania Department of Health morning confirmed three additional presumptive positive case of COVID-19 – two residents from Bucks County and one from Montgomery County. All are adults and in isolation at home. This brings the statewide total to 15 cases; 13 of the cases are presumptive positive and two cases, the Delaware County and Wayne County cases, have been confirmed by the CDC.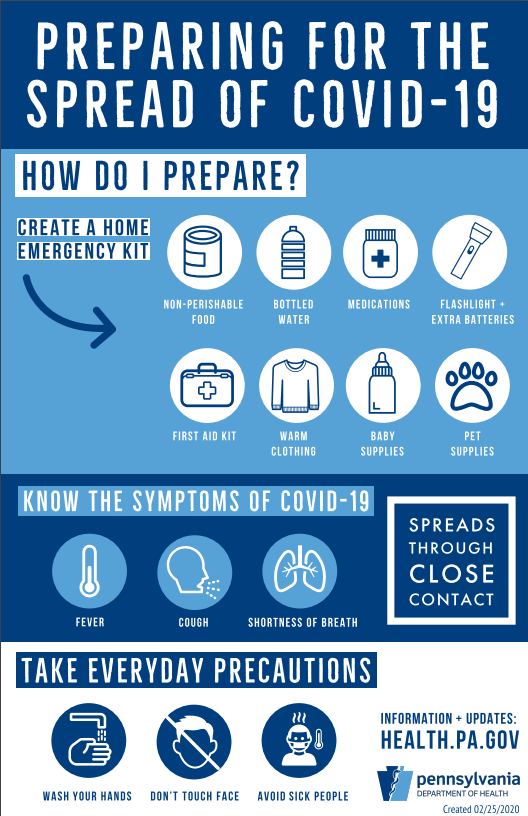
“While we anticipate that there will be more Pennsylvanians with COVID-19 in the coming days and weeks, it is important for residents to know the commonwealth is prepared and to be prepared themselves,” Secretary of Health Levine said. “Right now, you have a higher chance of testing positive for COVID-19 if you have traveled to a country or state with known community outbreaks or have come in contact with someone who has the virus. We are working with the health care community across Pennsylvania to keep them informed, consult on patient testing and ensuring they have the resources they need to care for patients.”
It is crucial to avoid spreading misinformation. Below are confirmed sources for accurate and factual information and updates.
Pennsylvania Department of Health
The Pennsylvania Department of Health is working diligently to monitor the COVID-19 situation in Pennsylvania and share information. Updates continue to be posted regularly DOH coronavirus webpage. The Department of Health is beginning daily press briefings on COVID-19 at noon every day. These briefings are livestreamed on PAcast. The link will be the same every day.
Center for Disease Control & Prevention (CDC)
The CDC COVID-19 webpage offers a wealth of information and resources on outbreak in the United States. Information on the disease, situation updates, and specific information for travel and professionals is available and updated as needed. The Pennsylvania Department of Health works in collaboration with the CDC for factual material.
Ready PA
Ready PA has the information to prepare by learning about:
- Different kinds of emergencies
- How to create all hazards/emergency plans and kits for your home, vehicle, and workplace
- How to plan ahead if you have a special need
The Wolf Administration Preparedness Actions
The World Health Organization first announced the coronavirus outbreak in late January and the Pennsylvania Department of Health has had its Emergency Operations Center set up since February 1. The center allows for a collaborative, concentrated state response, including:
- Activated the Department of Health’s Emergency Operations Center to allow for enhanced response coordination;
- Begun testing for COVID-19 at the state laboratory;
- Maintained communication and outreach with federal, state and local partners;
- Provided symptom monitoring for residents returning from areas impacted by coronavirus;
- Provided health care providers, businesses and education providers with information;
- Reviewed and adapted current pandemic flu plans to prepare for spread of COVID-19;
- Increased testing capacity;
- Partially activated the Commonwealth Response Coordination Center at PEMA.
- Governor Tom Wolf signed an emergency disaster declaration March 6 to ensure state agencies involved in the response have the expedited resources they need to continue to focus on the virus and its possible spread.
- The Department of Health is providing a daily update via statewide press release.
- On March 9, Sec. of Health Dr. Rachel Levine began to provide daily press briefings.
- CDC confirmed two cases, one in Delaware County and one in Wayne County. This means Pennsylvania has 13 presumptive positive cases and two positives.
CMS Announces Actions to Address Spread of Coronavirus

March 5, 2020
Today, the Centers for Medicare & Medicaid Services (CMS) is announcing several actions aimed at limiting the spread of the Novel Coronavirus 2019 (COVID-19). Specifically, CMS is issuing a call to action to health care providers across the country to ensure they are implementing their infection control procedures, which they are required to maintain at all times. Additionally, CMS is announcing that, effective immediately and, until further notice, State Survey Agencies and Accrediting Organizations will focus their facility inspections exclusively on issues related to infection control and other serious health and safety threats, like allegations of abuse – beginning with nursing homes and hospitals. Critically, this shift in approach, first announced yesterday by Vice President Pence, will allow inspectors to focus their energies on addressing the spread of COVID-19.
As the agency responsible for Medicare and Medicaid, CMS requires facilities to maintain infection control and prevention policies as a condition for participation in the programs. CMS is also issuing three memoranda to State Survey Agencies, State Survey Agency directors and Accrediting Organizations – to inspect thousands of Medicare-participating health care providers across the country, including nursing homes and hospitals.
“Today’s actions, taken together, represent a call to action across the health care system,” said CMS Administrator Seema Verma. “All health care providers must immediately review their procedures to ensure compliance with CMS’ infection control requirements, as well as the guidelines from the Centers for Disease Control and Prevention (CDC). We sincerely appreciate the proactive efforts of the nursing home and hospital associations that have already galvanized to provide up-to-the-minute information to their members. We must continue working together to keep American patients and residents safe and healthy and prevent the spread of COVID-19.”
The first memorandum released today provides important detail with respect to the temporary focus of surveys on infection control and other emergent issues. Importantly, it notes that, in addition to the focused inspections, statutorily-required inspections will also continue in the 15,000 nursing homes across the country using the approximately 8,200 state survey agency surveyors. Surveys will be conducted according to the following regime:
- All immediate jeopardy complaints (a situation in which entity noncompliance has placed the health and safety of recipients in its care at risk for serious injury, serious harm, serious impairment or death or harm) and allegations of abuse and neglect;
- Complaints alleging infection control concerns, including facilities with potential COVID-19 or other respiratory illnesses;
- Statutorily required recertification surveys (Nursing Home, Home Health, Hospice, and ICF/IID facilities);
- Any re-visits necessary to resolve current enforcement actions;
- Initial certifications;
- Surveys of facilities/hospitals that have a history of infection control deficiencies at the immediate jeopardy level in the last three years;
- Surveys of facilities/hospitals/dialysis centers that have a history of infection control deficiencies at lower levels than immediate jeopardy.
The memorandum also includes protocols for the inspection process in situations in which COVID-19 is identified or suspected. These protocols include working closely with CMS regional offices, coordinating with CDC, and other relevant agencies at all levels of government. The agency is also providing key guidance related to inspectors’ usage of adequate personal protective equipment.
The other two memoranda provide critical answers to common questions that nursing homes and hospitals may have with respect to addressing cases of COVID-19. For example, the memoranda discuss concerns like screening staff and visitors with questions about recent travel to countries with known cases and the severity of infection that would warrant hospitalization instead of self-isolation. They detail the process for transferring patients between nursing homes and hospitals in cases for which COVID-19 is suspected or diagnosed. They also describe the circumstances under which providers should take precautionary measures (like isolation and mask wearing) for patients and residents diagnosed with COVID-19, or showing signs and symptoms of COVID-19.
Finally, the agency is announcing that it has deployed an infection prevention specialist to CDC’s Atlanta headquarters to assist with real-time in guidance development.
Today’s actions from CMS are focused on protecting American patients and residents by ensuring health care facilities have up-to-date information to adequately respond to COVID-19 concerns while also making it clear to providers that as always, CMS will hold them accountable for effective infection control standards. The agency is also supplying inspectors with necessary and timely information to safely and accurately inspect facilities.
To view each memo, please visit the below links:
Suspension of Survey Activities: https://www.cms.gov/medicareprovider-enrollment-and-certificationsurveycertificationgeninfopolicy-and/suspension-survey-activities
Guidance for Infection Control and Prevention Concerning Coronavirus Disease (COVID-19): FAQs and Considerations for Patient Triage, Placement and Hospital Discharge: https://www.cms.gov/medicareprovider-enrollment-and-certificationsurveycertificationgeninfopolicy-and/guidance-infection-control-and-prevention-concerning-coronavirus-disease-covid-19-faqs-and
Guidance for Infection Control and Prevention of Coronavirus Disease 2019 (COVID-19) in nursing homes: https://www.cms.gov/medicareprovider-enrollment-and-certificationsurveycertificationgeninfopolicy-and/guidance-infection-control-and-prevention-coronavirus-disease-2019-covid-19-nursing-homes
Contact: CMS Media Relations
(202) 690-6145 | CMS Media Inquiries
