- Biden-Harris Administration Takes Historic Action to Increase Access to Quality Care, and Support to Families and Care Workers
- Biden Administration Sets Higher Staffing Mandates. Most Nursing Homes Don't Meet Them.
- Rural Jails Turn to Community Health Workers To Help the Newly Released Succeed
- Rural Communities Face Primary Care Physician Shortage
- Miles for Milk: How Student-Run Grocery Store Reshaped Rural Community's Food Access
- Native Americans Have Shorter Life Spans, and It's Not Just Due to Lack of Health Care
- Across the Country, Amish Populations Are on the Rise
- Using Medicaid to Address Young People's Mental Health Needs in School Settings
- Promotoras Play Essential Role in Connecting Farmworkers with Health Care in Rural NorCal
- Sunsets, Wildlife and Limited Care: Challenges of Aging in Place in Rural America
- City-Country Mortality Gap Widens amid Persistent Holes in Rural Health Care Access
- Tribal Environmental Impact Network
- Minnesota's Rural Ambulance Providers Look to State Capitol for Their Own Lifeline
- Biden-Harris Administration Takes Action to Support the Primary Care Workforce
- Over 3,000 Homes on the Navajo Nation Receive Accurate Addresses for the First Time
COVID-19 Disease Continues Steady Spread in Rural Areas from April 5-9
Daily Yonder, By Bill Bishop
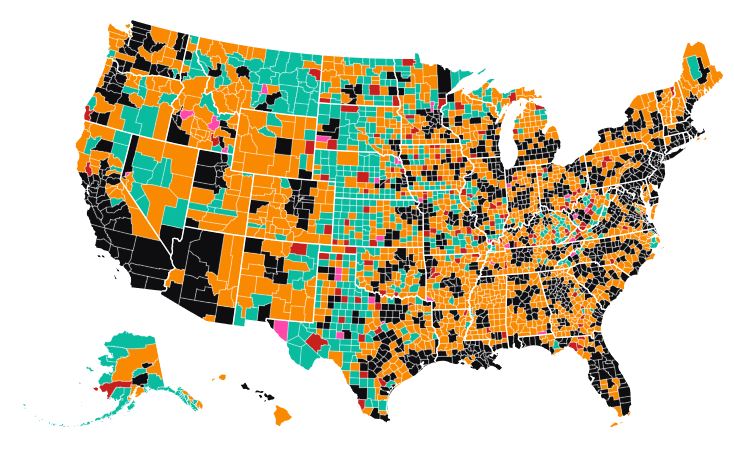
Nearly three out of every four rural counties have officially reported having a case of COVID-19 by the end of Thursday, according to data compiled from state health care agencies by USA Facts.
The map shows the spread of the novel coronavirus through rural America. The 130 rural counties in red reported their first case of COVID-19 between Sunday and Thursday, April 5-9, 2020. Only 31 counties (out of 1,164) metro counties have yet to report their first case of the virus as of the end of Thursday (April 9).
As of April 9, there have been 563 deaths from the virus in rural America. For the past week, the number of deaths from COVID-19 in rural counties has increased 12 to 17 % each day. Deaths in rural counties are increasing at about the same rate as the nation as a whole.
Deaths have been increasing the fastest in the suburbs of the nation’s major metropolitan areas, those with over a million people. Rural America still has a per capita rate of infection that is just a quarter of the national rate.
Click on the map and you can find data for your county. We have included the number of confirmed cases of COVID-19 and the number of deaths as of April 9.
Within the national picture of spreading cases and deaths are local stories that explain why some places have suffered more from the virus than others. For instance, we noticed that Mitchell County, Georgia, reported 12 deaths between Sunday and Thursday. News reports tells us that there was an outbreak of the virus at a nursing home, infecting 26 residents and killing nine in the last week.
There was also an outbreak at a Tyson Foods plant in Camilla, the county seat. Two Tyson workers have died after testing positive for COVID-19, according to the Retail, Wholesale and Department Store Union, which represents workers at the plant. Many workers at the Camilla plant commute from Albany, Georgia, which has had a large outbreak of the virus stemming from a funeral.
Kent County, Delaware, reported the largest number of new cases of COVID-19 in the last week of any rural county, with 128. It was followed by Litchfield County, Connecticut, with 118 and Sumter County, Georgia, with 111.
The COVID-19 pandemic is a world-wide event, but every community is experiencing it differently. Please tell us what’s happening in your community in the comments below or on our Facebook page. We can learn from each other.
USDA Unveils Tool to Help Rural Communities Address the COVID-19 Pandemic
USDA’s COVID-19 Federal Rural Resource Guide Lists Federal Programs That Can Help Rural Communities, Organizations and Residents Impacted by COVID-19
WASHINGTON, April 13, 2020 – U.S. Secretary of Agriculture Sonny Perdue today unveiled a one-stop-shop of federal programs that can be used by rural communities, organizations and individuals impacted by the COVID-19 pandemic. The COVID-19 Federal Rural Resource Guide (PDF, 349 KB) is a first-of-its-kind resource for rural leaders looking for federal funding and partnership opportunities to help address this pandemic.
“Under the leadership of President Trump, USDA is committed to being a strong partner to rural communities preparing for and impacted by COVID-19,” Perdue said. “This resource guide will help our rural leaders, whether they are in agriculture, education, health care or any other leadership capacity, understand what federal assistance is available for their communities during this unprecedented time.”
USDA has taken many immediate actions to assist farmers, ranchers, producers, rural communities, and rural-based businesses and organizations impacted by the COVID-19 pandemic. For more information on these actions, visit www.usda.gov/coronavirus.
Pennsylvania Has the 3rd Least Affected Small Businesses Due to Coronavirus – WalletHub Study
With 35 percent of small business owners saying their business cannot survive more than three months in current conditions, WalletHub today released its report on the States with the Most Affected Small Businesses due to Coronavirus, along with accompanying videos.
To identify the states in which businesses are hit hardest by COVID-19, WalletHub compared the 50 states and the District of Columbia across 12 key metrics. Our data set ranges from the share of small businesses operating in highly affected industries to small-business credit conditions and the state’s small-business friendliness. Below, you can see highlights from WalletHub’s report and a Q&A with WalletHub analysts.
COVID-19 Impact on Small Business in Pennsylvania (1=Most Affected, 25=Avg.):
- 43rd – Share of Small Businesses Operating in High-Risk Industries
- 51st – Share of Small-Business Employees Operating in High-Risk Industries Among Total Small-Business Employees
- 35th – Share of Consumer Expenditures Related to High-Risk Industries
- 20th – Share of Businesses with E-commerce Sales Activity
- 36th – Business Vitality
- 37th – Average Annual Federal Small-Business Funding per GDP
- 30th – Small-Business Credit Conditions
- 33rd – Total Amount of Small-Business Loans per Small-Business Employee
To view the full report and your state’s rank, please visit:
https://wallethub.com/edu/states-with-the-most-affected-small-businesses-due-to-coronavirus/72977/
USDA Announces Loan Maturity for Marketing Assistance Loans Now Extended to 12 Months
Agricultural producers now have more time to repay Marketing Assistance Loans (MAL) as part of the U.S. Department of Agriculture’s implementation of the Coronavirus Aid, Relief, and Economic Security (CARES) Act of 2020. The loans now mature at 12 months rather than nine, and this flexibility is available for most commodities.
Eligible commodities include barley, chickpeas (small and large), corn, cotton (upland and extra-long staple), dry peas, grain sorghum, honey, lentils, mohair, oats, peanuts, rice (long and medium grain), soybeans, unshorn pelts, wheat, wool (graded and nongraded); and other oilseeds, including canola, crambe, flaxseed, mustard seed, rapeseed, safflower, sunflower seed, and sesame seed. Seed cotton and sugar are not eligible
Pennsylvania Issues 30 Licensing Waivers Allowing Professionals to Respond to COVID-19 Emergency
Harrisburg, PA – The Pennsylvania Department of State announced that it has issued 30 licensing waivers since March 17 to allow licensed professionals, facilities and trainees to respond to the COVID-19 disaster declaration.
“During this unprecedented emergency, the Department of State is committed to reducing as many burdens as possible for licensees to practice and serve Pennsylvanians,” Secretary of State Kathy Boockvar said. “We’ve included a wide spectrum of professionals in these temporary waivers, recognizing that each professional we can empower to help is another critical part of the solution to ending this crisis.”
Among the changes in place during the COVID-19 emergency:
- Licensed health care practitioners may provide services via telemedicine
- Temporary licenses for out-of-state health care practitioners will be expedited
- Extended all upcoming license renewal deadlines including healthcare and non-healthcare professionals
- Recently retired health care practitioners may temporarily reactivate their licenses more easily and without reactivation fees
- Suspended certain in-person continuing-education requirements to allow increased use of online or distance learning
- Authorized the use of electronic notarization and loosened restrictions on in person requirements for notaries handling estate documents such as wills, living wills, and powers of attorney, as well as other types
- Extended filing deadlines for charitable nonprofit organizations
“The Department of State is working with the governor’s office, the Department of Health and the Department of Human Services to identify additional requirements that can be suspended to give licensed professionals and others the flexibility they need during the COVID-19 pandemic,” Secretary Boockvar said.
The Department of State website will be updated regularly as additional requirement waiver information becomes available. Licensees with questions should contact their state licensing board via the email addresses on the Department of State website.
CMS Summary: April 10, 2020

- CMS Approves Approximately $34 Billion for Providers with the Accelerated/Advance Payment Program for Medicare Providers in One Week
- COVID-19: Dear Clinician Letter
- COVID-19: Non-Emergent, Elective Medical Services and Treatment Recommendations
- Quality Payment Program: MIPS Extreme and Uncontrollable Circumstances Policy in Response to COVID-19
- Multi-Factor Authentication Requirement Delayed for PECOS, I&A, and NPPES
- Open Payments: Pre-Publication Review and Dispute through May 15
- Supplier Education on Use of Upgrades for Multi-Function Ventilators
- Second Update to CR 11152 Implementation of the Skilled Nursing Facility (SNF) Patient Driven Payment Model (PDPM) — Revised
- Civil Rights, HIPAA, and COVID-19
- Medicare Advance Written Notices of Noncoverage — Revised
- Medicare Preventive Services — Revised
- Medicare Preventive Services Poster — Revised
- CMS Approves Approximately $34 Billion for Providers with the Accelerated/Advance Payment Program for Medicare Providers in One Week
- COVID-19: Dear Clinician Letter
- COVID-19: Non-Emergent, Elective Medical Services and Treatment Recommendations
- Quality Payment Program: MIPS Extreme and Uncontrollable Circumstances Policy in Response to COVID-19
- Multi-Factor Authentication Requirement Delayed for PECOS, I&A, and NPPES
- Open Payments: Pre-Publication Review and Dispute through May 15
Weekly COVID-19 Update from NIOSH
As part of the National Institute for Occupational Safety and Health’s (NIOSH) efforts to keep our stakeholders up to date on the CDC and NIOSH COVID-19 response, below is a summary of new information posted this week
Industry Specific Resources
- Airline, Airport, and Transit Worker Fact Sheets
Airline, airport, and transit workers may be at risk for exposure to COVID-19. CDC recommends steps to prevent exposure, which includes everyday actions to prevent the spread of respiratory illness. To learn more, fact sheets are available for airline, airport, and transit workers.
Small Businesses
- Prepare your Small Business and Employees for the Effects of COVID-19
CDC has developed guidance to help small businesses limit the economic and community impacts of an outbreak of COVID-19. This new guidance provides steps to protect employees and prepare small businesses for disruption. A fact sheet also outlines 10 steps small business employers can take now to protect their employees’ health.
Healthcare Workers
- Strategies for Optimizing the Supply of N95 Respirators
CDC is working with partners across the global supply chain to evaluate and respond to reported shortages in PPE, particularly N95 respirators. This week, CDC updated guidance on Strategies to Optimize the Supply of N95 Respirators and released an accompanying Summary for Healthcare Facilities. - Elastomeric Respirators for U.S. Healthcare Delivery During N95 Shortages
This recorded webinar provides an overview of respiratory protection and guidance surrounding supply shortages. It also provides information on infection prevention measures, strategies for optimizing the supply of N95 respirators, and a broad overview of the use of elastomeric respirators in healthcare. - Updated Personal Protective Equipment (PPE) Burn Rate Calculator
CDC designed the PPE Burn Rate Calculator to help healthcare and nonhealthcare systems, such as correctional facilities, track how quickly PPE will be used at those facilities. This week CDC updated the tool, so it can now calculate the average PPE consumption rate per patient. Facilities can enter the number of patients in their facility and track changes in PPE usage as the number of patients fluctuates.
To stay up to date on the response, please visit the COVID-19 webpage or sign up for the COVID-19 newsletter.
Rural Areas with Vacation Homes More Susceptible to Coronavirus Outbreaks
The Hill
Rural communities with vacation homes are experiencing outbreaks of the coronavirus at a faster pace than rural areas without seasonal housing, according to a new study, suggesting residents of big cities who flee to the countryside are bringing the virus with them.
The survey found that in rural counties where more than 25 percent of the housing units are inhabited only part time, the average number of COVID-19 cases per capita is more than twice as high as the number of cases in counties where a greater percentage of the population lives there year-round.
Rural communities with high levels of vacation homes even have higher rates of infection than urban counties.
Trump Administration Acts to Ensure U.S. Healthcare Facilities Can Maximize Frontline Workforces to Confront COVID-19 Crisis

FOR IMMEDIATE RELEASE
April 9, 2020
Contact: CMS Media Relations
(202) 690-6145 | CMS Media Inquiries
At President Trump’s direction, the Centers for Medicare & Medicaid Services (CMS) today temporarily suspended a number of rules so that hospitals, clinics, and other healthcare facilities can boost their frontline medical staffs as they fight to save lives during the 2019 Novel Coronavirus (COVID-19) pandemic.
These changes affect doctors, nurses, and other clinicians nationwide, and focus on reducing supervision and certification requirements so that practitioners can be hired quickly and perform work to the fullest extent of their licenses. The new waivers sharply expand the workforce flexibilities CMS announced on March 30.
CMS sets and enforces essential quality and safety standards for the nation’s healthcare system that supplement State scope-of-practice and licensure laws for healthcare workers. CMS has continuously examined its regulations to identify areas where Federal requirements may be more stringent than State laws and requirements. The changes CMS is announcing today will ensure that healthcare facilities across the nation can expand their staffs and organize them in the most efficient way possible to handle the incoming surge of COVID-19 patients.
Hospitals and health systems throughout the U.S. are seeing increases in patient volumes, leading to significant challenges in delivering vital services. Allowing clinicians to practice to the full scope of their licenses is critical to address staffing needs during the public health emergency.
As a result of CMS’s action:
- Doctors can now directly care for patients at rural hospitals, across state lines if necessary, via phone, radio, or online communication, without having to be physically present. Remotely located physicians, coordinating with nurse practitioners at rural facilities, will provide staffs at such facilities additional flexibility to meet the needs of their patients.
- Nurse practitioners, in addition to physicians, may now perform some medical exams on Medicare patients at skilled nursing facilities so that patient needs, whether COVID-19 related or not, continue to be met in the face of increased care demands.
- Occupational therapists from home health agencies can now perform initial assessments on certain homebound patients, allowing home health services to start sooner and freeing home-health nurses to do more direct patient care.
- Hospice nurses will be relieved of hospice aide in-service training tasks so they can spend more time with patients.
“It’s all hands on deck during this crisis,” said CMS Administrator Seema Verma. “All frontline medical professionals need to be able to work at the highest level they were trained for. CMS is making sure there are no regulatory obstacles to increasing the medical workforce to handle the patient surge during the COVID pandemic.”
CMS’s workforce changes apply immediately and address supervision, licensure and certification, and other limitations in healthcare settings including Critical Access Hospitals (CAHs), Rural Health Clinics (RHCs), Federally Qualified Health Centers (FQHCs), Skilled Nursing Facilities (SNFs), Home Health Agencies (HHAs), and Hospice. These actions are part of the unprecedented array of temporary regulatory waivers and new rules issued recently by CMS and intended to help the American healthcare system respond to COVID-19.
CMS is the nation’s largest health insurer, serving more than 140 million Americans through Medicare, Medicaid, the Children’s Health Insurance Program, and Federal Exchanges.
On March 30, CMS issued an unprecedented array of temporary regulatory waivers and new rules to allows hospitals and healthcare systems to deliver services at other community-based locations to make room for COVID-19 patients needing acute care in their main facilities. The changes complement and augment the work of FEMA and state and local public health authorities by empowering hospitals and healthcare systems to rapidly expand treatment capacity and separate infected from uninfected patients. CMS’s waivers and flexibilities will permit patients to be triaged to a variety of community-based locales, including ambulatory surgery centers, inpatient rehabilitation hospitals, hotels, and dormitories. Transferring uninfected patients will help hospital staffs to focus on the most critical COVID-19 patients, maintain infection control protocols, and conserve personal protective equipment (PPE).
In recent weeks, CMS also has temporarily:
- Permitted physicians whose privileges will expire to continue practicing at a hospital, and allowed new physicians to begin working prior to full hospital medical staff/governing body review and approval.
- Lifted regulatory requirements regarding hospital personnel qualified to perform specific respiratory care procedures, allowing these professionals to operate to the fullest extent of their licensure;
- Waived federal minimum personnel qualifications for clinical nurse specialists, nurse practitioners, and physician assistants so they can work at rural hospitals as long as they meet state licensure requirements, allowing for maximum staffing flexibility at such facilities
- Allowed physicians and non-physician practitioners to use telehealth technology to care for patients at long-term care facilities, rather than having to treat patients there in person.
- Waived certain training and certification requirements for nurse’s aides at long term care facilities, to help address potential staffing shortages during the pandemic;
- Waived paperwork requirements so that hospital doctors can use more verbal, rather than written medical orders;
For a complete list of workforce flexibilities that CMS has permitted in recent weeks and years, go to: https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
For a fact sheet detailing additional information on the waivers announced today and previously, go to: https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf
These actions, and earlier CMS actions in response to COVID-19, are part of the ongoing White House Coronavirus Task Force efforts. To keep up with the important work the Task Force is doing in response to COVID19, visit www.coronavirus.gov. For a complete and updated list of CMS actions, and other information specific to CMS, please visit the Current Emergencies Website.
CMS NEWS ALERT: April 9, 2020 (COVID-19)NEWS ALERT
April 9, 2020
Here is a summary of recent Centers for Medicare & Medicaid Services (CMS) actions taken in response to the COVID-19 virus, as part of the ongoing White House Task Force efforts. To keep up with the important work the Task Force is doing in response to COVID-19, click here www.coronavirus.gov. For information specific to CMS, please visit the CMS News Room and Current Emergencies Website. CMS updates these resources on an ongoing basis throughout the day; the information below is current as of April 9, 2020 at 2:30 p.m.
CMS Approves Over $51 Billion for Providers with the Accelerated/Advance Payment Program for Medicare Providers in One Week
CMS delivered more than $51 billion to the healthcare providers on the frontlines battling the 2019 Novel Coronavirus (COVID-19) to ensure they have the resources they need to combat this pandemic. This is an increase from the $34 billion that CMS announced in an earlier press release. Processing times for a request of an accelerated or advance payment is now four to six days, down from the previous timeframe of three to four weeks. In a little over a week, CMS has already approved over 21,000 of the 32,000 requests it received from health care providers and suppliers seeking accelerated and advance payments. Prior to COVID-19, CMS had approved just over 100 total requests in the past five years, with most being tied to natural disasters such as hurricanes.
Note: Payments are now up to $51 billion. CMS approved over 21,000 of 32,000 requests received.
CMS Issues New Wave of Infection Control Guidance to Protect Patients and Healthcare Workers from COVID-19
CMS issued a series of updated guidance documents focused on infection control to prevent the spread of the 2019 Novel Coronavirus (COVID-19) in a variety of inpatient and outpatient care settings. The guidance, based on Centers for Disease Control and Prevention (CDC) guidelines, will help ensure infection control in the context of patient triage, screening and treatment, the use of alternate testing and treatment sites and telehealth, drive-through screenings, limiting visitations, cleaning and disinfection guidelines, staffing, and more.
Coronavirus-Related Medicare Scam Alert Blog
The Social Security Administration featured a guest blog from CMS Administrator Seema Verma reminding Medicare beneficiaries to be vigilant and take precautions to avoid falling victim to healthcare fraud during the coronavirus pandemic.
Artificial Intelligence Health Outcomes Challenge Pause
CMS will temporarily pause the Artificial Intelligence Health Outcomes Challenge and restart the Challenge on Monday, June 29, 2020. In the coming weeks, CMS will distribute a more detailed timeline for the remaining stages of the Challenge.
Artificial Intelligence Health Outcomes Challenge
Emergency Triage, Treat, and Transport (ET3) Model Implementation Date Delay
As CMS seeks to support the community of organizations that are responding to the COVID-19 public health emergency, CMS will delay the start of the ET3 Model from May 1, 2020 until Fall 2020. Selected applicants have been notified and will be required to complete a revised Participation Agreement reflecting the new implementation date.
CMS Approves Additional State Medicaid Waivers and Amendments to Give States Flexibility to Address Coronavirus Pandemic
CMS continues to deliver urgent regulatory relief to ensure states can quickly and effectively care for their most vulnerable citizens during the COVID-19 crisis. For the first time, CMS has approved a COVID-related Children’s Health Insurance Program (CHIP) Disaster Amendment that brings relief for CHIP-covered children living in Maine. In addition, CMS approved COVID-related Medicaid Disaster Amendments that bring relief to North Dakota, Rhode Island, and Wyoming. These approvals help to ensure that states have the tools they need to combat COVID-19 through a wide variety of state plan flexibilities. CMS also authorized amendments to ensure emergency flexibilities in programs that care for the elderly and people with disabilities, including most recently in Delaware, Hawaii, Mississippi, New York, and North Dakota. These approved flexibilities support President Trump’s commitment to a COVID-19 response that is locally executed, state managed, and federally supported.
All told, CMS has approved 49 emergency waivers, 26 state amendments, 7 COVID-19 related Medicaid Disaster Amendments and the first CHIP COVID-related Disaster Amendment in record time. States are using a toolkit CMS developed to expedite the application and approval of Medicaid state waivers and State Plan Amendments.
Medicaid State Plan Amendments
1915(c) Appendix K Waivers
